Recent livestock disease investigations
Lumpy skin disease excluded in a dairy cow with skin lesions
- A private veterinarian was called to a property within the Perth metropolitan area to examine a 3 year-old Holstein-Friesian cow.
- Staff had noticed the cow to be uncomfortable during milking and found multiple well-defined plaques on the caudal teats and ventral udder. Lesions were not evident elsewhere on the cow.
- The cow was otherwise in good health, no history of mastitis and no discernible milk production drop. No other animals had similar lesions.
- The private vet contacted their local DPIRD field veterinary officer to arrange for laboratory testing, which was subsidised by DPIRD to rule out reportable diseases.
- Diseases considered on the differential list included: superficial bacterial infection, photosensitisation, chemical burns, bovine popular stomatitis, and bovine ulcerative/herpes mammalities and exotic disease, Lumpy skin disease (LSD).
- Samples collected from the live animal included fresh swabs for bacterial culture, fresh skin tissue for viral testing, serum and EDTA blood for antibodies and biochemistry and milk for bacterial culture.
- Diagnostic testing was run at DDLS and ACDP laboratories enabling exclusion of LSD, bovine papular stomatitis, pseudocowpox and bovine herpesvirus type 2 and 4.
- Staining of the skin biopsy and examination under the electron microscopy did not detect any viral particles.
- Bacteriology yielded Staphylococcus from a fresh plaque confirming the putative diagnosis of staphylococcal dermatitis.
- Testing helps to prove that WA is free from trade sensitive reportable diseases and further supports the producer and industry by confirming the diagnosis and enabling prevention programs and increased productively. The Significant Disease Investigation program boosts WA’s capacity for early detection by subsidising the cost of investigating diseases. For more information or approvals, contact your local DPIRD field veterinarian. If your case does not fall under the SDI program eligibility criteria, contact the DDLS on-duty pathologist on 08 9368 3351 to discuss lab-exempt testing for reportable disease exclusions.
Australia continues to be vigilant in surveillance for LSD to support Australia’s free status that underpins access to many important cattle markets. If you are concerned about skin lesions during an investigation please contact:
- the EAD Hotline on 1800 675 888
- your local DPIRD field veterinarian or
- the DDLS on-duty pathologist on 08 9368 3351 to report your observations and assistance with sampling and testing.
See DPIRD’s webpage on Lumpy skin disease (LSD) for more information.
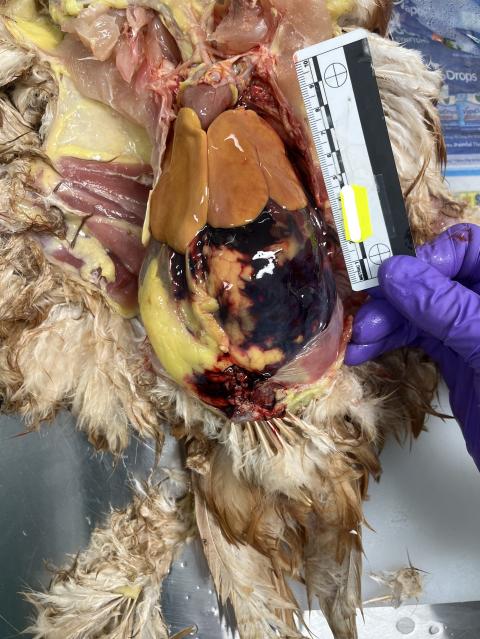
EAD Hotline alerted DPIRD field veterinarians to deaths in backyard poultry
- A sheep and cropping producer phoned the EAD Hotline to notify DPIRD veterinarians of ongoing deaths in their backyard chicken flock.
- The chickens were dying sporadically over a few weeks with clinical signs of lethargy, isolating themselves, pale coloured combs and fluffed up appearance. There was also a mild egg production drop from the flock.
- No other livestock species were affected. Recent environmental changes included baiting for mice and spraying the chicken coop with disinfectant.
- A property visit was organised to view the sick and recently deceased birds. On examination, the DPIRD field veterinarian noted moderate respiratory signs, mild purulent bilateral nasal discharge, green discolouration of the dorsal neck skin and purple discolouration on the tips of the comb.
- One carcass was submitted to DDLS laboratory for post-mortem.
- On necropsy, there was extensive acute haemorrhage which was consistent with poor clotting capacity as a result of rodenticide exposure. Although baits were not directly accessible to the chickens, secondary toxicity from consuming poisoned mice was a possible scenario.
- Reportable diseases Avian Influenza (AI) and Newcastle Disease (ND) were excluded.
- Tracheal and cloacal swabs are required to be collected to perform exclusion testing for reportable diseases in poultry, such as AI and ND. Firmly swab the cloaca and trachea mucosa with dry swabs, then snap swab heads into separate viral transport media (VTM) for avian influenza and Newcastle disease virus testing.
- VTM is available from your local DPIRD veterinary officers.
For more information, see DPIRD’s chicken necropsy guide.
In late spring/ summer, be on the lookout for:
| Disease, typical history and clinical signs | Samples (additional to base set) |
|---|---|
Enterotoxaemia (pulpy kidney) in lambs
Read more on enterotoxaemia. | Ante-mortem:
Post-mortem:
Adult sheep may be eligible for a TSE exclusion and subsidy. |
Haemonchosis in sheep (barber's pole worm)
Read more on barber's pole worm. | Ante-mortem:
Post-mortem:
|
Selenium deficiency in lambs and calves
| Ante-mortem:
Post-mortem:
|
Also include base samples and any clinical or gross lesions in submissions. For advice on sample submission, contact your DPIRD field veterinary officer, the sampling and post-mortem resources webpage (see below) or phone the duty pathologist on +61 (0)8 9368 3351.
See DPIRD’s webpages for the livestock disease veterinary sampling guide and veterinary sample packaging guide.
Significant disease investigation program summary for the 2022-2023 financial year
A total of 171 significant disease investigations were completed by private veterinarians from 27 practices in the last financial year from July 2022 to June 2023.
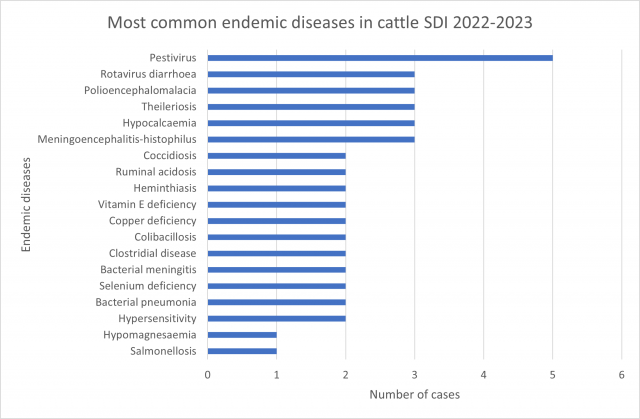
The Significant Disease Investigation (SDI) program remains central to providing the data required for early disease detection and to underpin health certification for export market access. Nearly all the cases confirmed an endemic diagnosis that the vet could assist the producer to manage, highlighting the benefit of the program to producers.
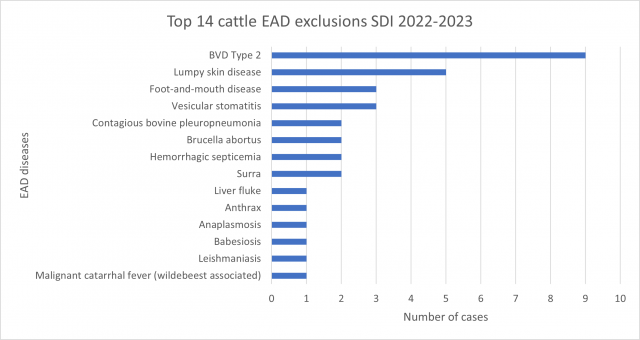
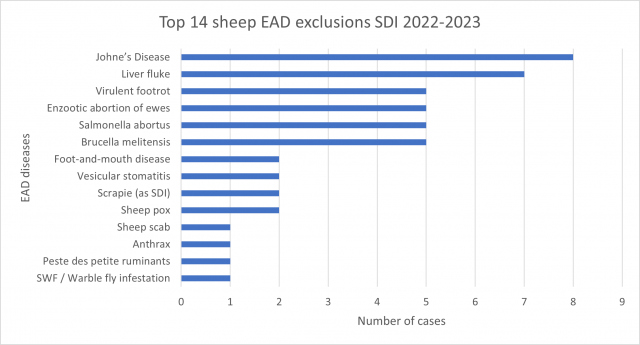
Of the 171 cases submitted by private veterinarians, 54 cases (31.6%) submissions were suitable for emergency disease exclusions. Another 8 cases (4.7%) returned a positive diagnosis for a reportable endemic disease, all were Johne’s disease in sheep. Of the 54 cases clinically similar to an emergency animal disease, 80 exotic diseases were tested for and excluded*.
*Some submissions were tested for more than one emergency disease.
| Species | SDI submissions |
|---|---|
| Ovine | 102 |
| Bovine | 66 |
| Caprine | 3 |
| Porcine | 0 |
| Camelid | 0 |
The program demonstrates geographic reach** and there has been a year-on-year increase in the number of veterinarians and practices engaging with the program.
**northern Australia is covered by a separate program.
Most common EAD exclusions performed during 2022-2023
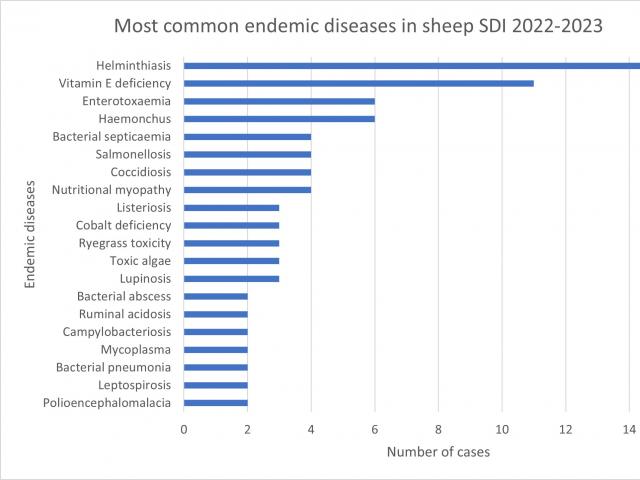
Most common endemic diseases diagnosed 2022-2023
Refresher on base sample sets
Correct post-mortem and sampling will increase the likelihood of a definitive diagnosis. Make sure to include a detailed history including owner name and PIC, provide a timeline and spatial distribution of the diseased stock, information about the stock including age, breed, sex and numbers in the mob, number affected and number dead with a description of the clinical signs, post mortem findings and a description of lesion distribution, size, shape and colour on the DPIRD Diagnostic Laboratory Services (DDLS) submission form. Taking photos of the lesion and emailing to DDLS is helpful.
A full set of samples is required for cases submitted through the Significant Disease Investigation (SDI) program. DPIRD provides a subsidy of $330 (GST inclusive) to private veterinarians as well as waiving all laboratory costs for an initial field and laboratory investigation of significant disease incidents in livestock approved under the SDI program.
See the SDI program webpage for information on eligibility criteria and approval processes. SDI sample kits can be replaced after use for an SDI by your local DPIRD veterinary officer.
For more information or help with sample for specific diseases or syndromes, contact your local DPIRD field veterinary officer or the DDLS on-duty pathologist on 08 9368 3351.
| Sample type | Sample to collect | Storage/transport |
|---|---|---|
Antemortem samples | Lithium heparin 10 mL EDTA 10 mL Clotted 10 ml Faeces 10 g | Blood samples:Mix with anticoagulant and chill; do not freeze. Draw off serum if prolonged transport. |
Fresh tissue(individual containers) | Brain (swab/ small section) Lung Liver Kidney Rumen contents 100-200 g Ileal content 10 mL Vitreous humour 1 mL Faeces/ rectal content 10 g | Fresh samples:Chill / refrigerate; do not freeze. |
Fixed samples(pooled in formalin – 10:1 formalin:tissue) | Brain Lung Heart Liver Kidney Rumen Abomasum Small intestine Large intestine Skeletal muscle | Fixed tissues:Keep at room temperature |
DPIRD has produced visual guides to assist in carrying out thorough post-mortems in a range of livestock species. See the DPRID webpages for more information on the Livestock disease veterinary sampling guide, as well as post-mortems in ruminants, chickens and pigs. Further resources can be found on the Sampling and post-mortem resources for veterinarians webpages.
Livestock disease investigations protect our markets
Australia’s ability to sell livestock and livestock products depends on evidence from livestock disease surveillance and investigations to provide evidence Australia is free from many significant livestock diseases that affect trade.
Find out more about WA's animal health surveillance programs.
Feedback
We welcome feedback. To provide comments or to subscribe to the monthly newsletter, email waldo@dpird.wa.gov.au. To see previous issues of the WALDO – for vets, please visit our archive page. Producer versions of this newsletter are available on the WALDO - for producers archive page.