The unknown "Dongara" weevil has been attacking canola
- Mingenew
- Dongara
- Mount Budd


An unknown pest weevil has been reported damaging a canola crop near Mingenew by Mingenew Irwin Group (MIG) Project officer Jacqui Meares. Tiny adult weevils were found chewing on the hypocotyl at or just below the soil surface. They are dark brown to black in appearance without prominent markings, approximately 3-5 mm long, and are flightless. They survived typical rates of insecticide used for common pests of canola and are easily confused with desiantha weevils.
Only adults have been found to date and their lifecycle remains unknown. The weevil has previously been reported causing extensive damage in canola and coriander seedling crops grown in heavy fine cracking clay soils in the Mingenew and Dongara areas. It has been dubbed the Dongara weevil referring to the first place from which it was reported in 2013.
The Dongara weevil mostly feeds at night and is elusive during the day usually sheltering in clusters at the base of seedlings or under clods or in deep cracks in heavy fine clay soils. This behaviour is common with many other weevil pests.
The Dongara weevil is smaller and darker in colour than the four species of weevils commonly found damaging canola, vegetable weevil, desiantha (spotted vegetable) weevil, small lucerne weevil and Fuller’s rose weevil. For more information refer to the 2023 PestFacts WA Issue 3 article Identifying weevils.
A joint investigation of the new weevil pest is being undertaken by researchers from DPIRD, MIG and Murdoch University. This research is being co-funded by GRDC and DPIRD. An understanding of the weevil’s host and environmental preferences, along with taxonomic classification will assist with the development of effective management strategies including tools and information to correctly identify the pest.
Surveillance will occur in the 2023 and 2024 seasons, using pitfall traps and opportunistic sampling in paddocks within a 50 km radius of the known Dongara weevil sites. Recently Research scientist Christiaan Valentine (DPIRD) has been tracking the weevil at night with fluorescent powder and a UV light to see how it moves and where it goes.
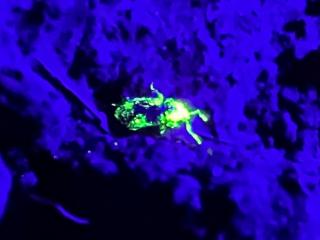
A taxonomic species description and molecular analysis will be prepared by DPIRD and Murdoch University researchers. It has been confirmed by international weevil specialists that the Dongara weevil does not match any known weevil species worldwide, and it is unlikely to be a native species. The Dongara weevil belongs to the family Curculionidae, the largest insect family in the world with over 300 000 species, and less than one third named in Australia.
Managing Dongara weevils
Preliminary testing of broad-spectrum insecticides have shown that most insecticides registered for weevils in canola performed poorly. Research scientist Dr Dusty Severtson (DPIRD) suggests that the weevils are using avoidance behaviour to evade synthetic pyrethroids such as alphacypermethrin and organophosphates such as chlorpyrifos. He noted that weevils were often found hiding just below the soil surface within furrows at the base of seedlings away from foliar spray exposure.
The registered rate for vegetable weevil, alphacypermethrin at 400 ml/Ha, performed poorly on the Dongara weevil.
Damaged canola crops had also been treated with a neonicotinoid seed dressing and this seems to not bother the weevils at all after being seen feeding on the tiny seedlings.
For more information on weevils visit DPIRD’s Diagnosing weevils in canola and Diagnosing Desiantha weevils in cereals pages.
For more information contact Research scientist Christiaan Valentine, Northam on +61 (0)8 9690 2197.
Article author: Bec Severtson (DPIRD Northam).
European earwigs are hatching again
- Ongerup
- South Stirlings
- Wellstead
A second hatching of European earwigs has recently occurred in crops at Ongerup, South Stirlings and Wellstead.
Growers will need to vigilant for European earwig activity next season, particularly in paddocks being sown to canola.
Adult European earwigs range from 12-24mm long. They have uniform brown bodies that are smooth and shiny with light brown/ yellow legs, pincers (also called forceps) and 'shoulders'.
European earwigs should not be confused with native earwigs. European earwigs tend to be found communally whereas native earwigs are usually solitary.
When checking crops look for shredded leaf tips, or jagged holes in the leaves as this is typical of earwig damage. In severe situations European earwigs can completely defoliate young seedlings leaving only stems or bare ground in patches. This pest is hard to find during the day and it is best to look for European earwigs at night using a torch or by placing pitfall traps into the ground.
Insecticides especially chlorpyrifos or alphacypermethrin applied at the highest registered rates do have efficacy against European earwig as a contact only. However European earwigs can be difficult to control as they hide under stubbles. Sprays applied at night have worked better than sprays applied during the day as earwigs are more active at night. Baiting for European earwigs has provided better control for this pest than spraying, especially in paddocks with heavy stubble loads. Baits are a better option as sprays will also control any predators such as carabid beetles. These predators play a role in suppressing pest populations and reducing broad-spectrum spray applications will increase their numbers.
To read about earlier European earwig activity this season refer to the 2023 PestFacts WA Issue 2 article European and native earwigs.
For more information on this pest refer to DPIRD’s Diagnosing European earwig and Management of European earwig pages.
For more information contact Research scientist Svetlana Micic, Albany on +61 (0)8 9892 8591.
Article authors: Cindy Webster (DPIRD Narrogin) and Svetlana Micic (DPIRD Albany).
Blackleg in canola
As early sown canola crops start to come into flower across the grainbelt growers are urged to consider management of blackleg upper canopy infection as necessary.
What is blackleg?
Blackleg, caused by the fungus Leptosphaeria maculans, is one of the most serious diseases of canola in Western Australia. Blackleg can cause significant damage by infecting the cotyledons, and the first leaves when plants are seedlings, leading ultimately to crown lesions or cankers later in the season. The spores can also infect the crop in the late vegetative and flowering stage resulting in upper canopy infection (UCI). Of all the various forms of upper canopy infections, early stem and major branch infections are the most damaging to yield.

Blackleg is primarily spread by ascospores on the wind, with the heaviest spore fall out occurring within 500 metres of any canola residue. Each year, canola residue continues to produce blackleg spores at a diminishing rate until the stubble has completely broken down.
The overall risk of blackleg infection on a property will be determined by factors such as choice of variety resistance group (and frequency of variety use), paddock rotation, time of sowing, fungicide usage, distance from previous year’s canola residues, and stubble management.
To minimise the risk of blackleg infection, growers should consider:
- Sowing into paddocks that are out of canola rotation for more than three years.
- Avoiding sowing within 500m of last year’s canola residues.
- Applying seed dressing and fertiliser applied fungicide. Reduced sensitivity to Jockey® and flutriafol has been reported in some WA blackleg populations. Please report any unexpected failures to your agronomist or DPIRD.
- Applying a foliar seedling fungicide in case of high disease pressure. You can assess your risk level by referring to GRDC’s Blackleg Management Guide 2023 Autumn Fact Sheet, and DPIRD’s BlacklegCM decision support tool.
For more information, refer to DPIRD’s Diagnosing blackleg in canola page.
Managing blackleg infection
For both blackleg stem canker and blackleg UCI, growers need to consider their varietal resistance levels before they spray as it may not be economical to spray varieties with high resistance levels.
Fungicides applied during the bloom stage may reduce UCI, and there are now a range of products registered for use. These products may be applied at similar times to the fungicides used for sclerotinia control. These fungicide applications for UCI are likely to be more economical in higher yielding crops.
For more information regarding canola variety resistance ratings and blackleg management, refer to GRDC’s Blackleg Management Guide 2023 Autumn Fact Sheet, and DPIRD’s Registered foliar fungicides for canola in WA page.
Deciding on your best course of action
DPIRD’s UCI BlacklegCM app will help you decide if spraying of your paddock is economical. UCI BlacklegCM takes into account costs of production, seasonal weather information, crop history, presence of blackleg infected leaves and fungicide strategies to give you the best case, worst case and most likely estimates of financial return from different management strategies for blackleg UCI. For more information refer to DPIRD’s UCI BlacklegCM page.
Canola blackleg risk forecasts
DPIRD's blackleg spore maturity forecasts for Western Australia for the 2023 growing season are available online. The latest forecast is current to 19 June 2023.
The forecasts show the expected risk during the 4-6 leaf stage, relative to the date of sowing. For crops sown in mid to late May, the risk of blackleg spore showers coinciding with the seedling susceptible stage is high at most locations within the state.
For more information, refer to DPIRD’s Canola blackleg spore maturity forecast for Western Australia page to check the blackleg model forecast for your district.
More information
For more information about blackleg in canola contact Research scientist Andrea Hills, Esperance on +61 (0)8 9083 1144.
For more information about the blackleg risk forecast, or the UCI BlacklegCM decision support tool, contact Senior research scientists Jean Galloway, Northam on +61 (0)8 9690 2172 or Adam Sparks, Perth on +61 (0)8 9368 3689.
Article authors: Jean Galloway (DPIRD Northam) and Cindy Webster (DPIRD Narrogin).
Article input: Andrea Hills (DPIRD Esperance) and Adam Sparks (DPIRD Perth).
Sclerotinia stem rot
- Geraldton
- Northam
- Albany
Research scientist Zia Hoque (DPIRD) has reported finding basal sclerotinia infection on Jurien lupins in an irrigated inoculated DPIRD trial at Northam. The plants were at the 10-12 leaves stage.

Sclerotinia on lupins is caused by Sclerotinia sclerotiorum, the same pathogen that causes sclerotinia stem rot in canola.
Technical Officer Anne Smith (DPIRD) has reported finding apothecia (germinated from sclerotia) at Geraldton under April sown canola plants in a trial that was irrigated at sowing. The hybrid canola variety has a leafy dense canopy and has been remaining moist through the day favouring the germination of sclerotia.
Apothecia have also been observed in the sclerotia depots at Northam and Albany this week. It is a warning that the cool moist weather in recent weeks has suited the commencement of the sclerotinia disease lifecycle.
Growers in areas with a history of sclerotinia are reminded to monitor their lupin and canola crops for sclerotinia stem rot infection and consider sclerotinia management when their crops are close to or at flowering.
Symptoms
Sclerotinia canopy infection is the most common infection pathway with aerial ascospores produced by apothecia infecting petals during crop flowering. This is known as carpogenic germination of sclerotia. Lesions occur in the upper half of the lupin’s main stem or branches and on pods. Basal sclerotinia is a different infection pathway where sclerotia germinate myceliogenically and directly infect the stem at ground level in very wet conditions/seasons. The fungus moves through the soil and produces a white cottony-looking growth that girdles the stem, causing the plant parts above the lesion to wilt and die. Hard black sclerotia, 2-8 millimetres (mm) in diameter, are produced in the fungal growth or in the cavities of infected stems or pods. Sclerotia can survive in soil for several years and are the source of new sclerotinia infections for all broadleaf crops and pastures.
Management
Research to date has shown that basal sclerotinia is very difficult to manage as the fungal infection (directly from sclerotia) is occurring at or below ground level. The lupin sclerotinia project is investigating some potential strategies but currently no foliar fungicides are registered or recommended for reducing basal sclerotinia infection in canola or lupin.
DPIRD research has shown that regular rainfall and high humidity (>75%) in the three weeks before and after commencement of flowering are most conducive for damaging levels of canopy sclerotinia to occur in crops. While fungicide application reduces levels of canopy infection, it does not necessarily give a yield response, so it’s important to consider crop risk and value of disease management carefully each season.
Growers need to consider the following factors to determine their risk of sclerotinia and which paddocks to prioritise:
- rotation history of the paddock
- history of sclerotinia in the current paddock and those surrounding it
- rainfall events before and after flowering
- crop growth stage
- dense crops with early canopy cover on loamy soil types are at higher risk.
The SclerotiniaCM decision support tool is available for use by canola growers during flowering to help determine the likely economic returns from applying fungicide at a specific time during flowering for the control of sclerotinia stem rot. The user can specify individual paddock data/history as well as recent and expected weather conditions so that the output relates to their own cropping circumstances. The SclerotiniaCM tool can be downloaded from the App Store or Google Play Store and can be used on both phones and tablet devices. For more information refer to DPIRD’s SclerotiniaCM decision support tool page.
Several fungicide products are registered for the control of canopy sclerotinia in canola while options in lupin are more limited. Fungicides need to be applied as recommended per product label. Strategic and responsible use of fungicides will reduce the risk of fungicide resistance developing. For more information refer to DPIRD’s Registered foliar fungicides for canola in WA and Registered foliar fungicides for lupin crops in WA pages.
Based on the extensive research conducted by DPIRD the following in-season sclerotinia management options are:
- For canola:
- Apply a single foliar application at 30-50% bloom, provided conditions are favourable for infection before and during flowering. See Table 1 below for recognising bloom stages in canola. Fungicides cannot be applied after 50% bloom (full bloom or when crop at its brightest). Use the SclerotiniaCM tool for guidance.
- A second fungicide application at 50% bloom is generally only beneficial in seasons with an extended wet period. Use the SclerotiniaCM tool for guidance.
- For lupin:
- Aim to apply fungicide from full to late flowering on main spike in order to protect main stem pods and penetrate the lower canopy
- A range of products are now registered in lupins which can reduce sclerotia canopy infection e.g., Veritas® Opti and Miravis® Star.
| Percent bloom | Number of flowers open on the main stem |
|---|---|
| 5% | <5 |
| 10% | 10 |
| 20% (petal drop commences) | 11-14 |
| 30% | 15-20 |
| 50% (full bloom, crop is at its brightest) | >20 |
Growers and consultants are encouraged to report to the PestFacts WA service any apothecia finds or disease observations as the season progresses.
Further information can be found at;
- DPIRD’s Managing sclerotinia stem rot in canola page
- DPIRD’s Managing sclerotinia in lupins page
- GRDC’s Sclerotinia stem rot in canola factsheet.
For more information on Sclerotinia in canola contact plant pathologists Andrea Hills, Esperance on +61 (0)8 9083 1144, Ciara Beard, Geraldton on +61 (0)8 9956 8504, Kylie Chambers, Northam on +61 (0)8 9690 2151 or Jean Galloway, Northam +61 (0)8 6690 2172.
For more information on sclerotinia in lupins contact plant pathologists Ciara Beard, Geraldton on +61 (0)8 9956 8504 or Geoff Thomas, South Perth on +61 (0)8 9368 3262.
Article authors: Ciara Beard (DPIRD Geraldton), Jean Galloway (DPIRD Northam) and Andrea Hills (DPIRD Esperance).
Article input: Zia Hoque (DPIRD Northam).
Early spot-form net blotch is around, but stay calm and don’t spray
- Wialki
- Kalannie
- Moorine Rock
- Merredin
- North Lake Grace
- Beaumont
- Gibson
Growers and agronomists may have noticed that their barley crops have some spot-form net blotch (SFNB) lesions starting to appear.
Initially SFNB lesions are dark brown and tend to be rounded. In contrast, early net-form net blotch (NTNB) lesions, while also dark brown, tend to start as very thin, rectangular ‘nets’.
Plant pathologist Jason Bradley (DPIRD) has had barley on barley field trials in the central region that highlight the effectiveness of variety resistance. The trials have shown that Maximus CL, while equally likely to have early SFNB as Spartacus CL during tillering, becomes slightly more resistant as the plant ages so that SFNB develops more slowly as the plant elongates (Figure 1). Jason is currently trialling the SFNB response of newer varieties with improved resistance as a management strategy in environments where finishing rains are not always guaranteed.

Analysis of over 30 SFNB management trials in low rainfall areas has shown that even when SFNB is present early, tillering sprays are not useful in delivering an economic return. Unnecessary fungicide applications being applied as part of a tank-mix could actually be making the evolution of fungicide resistance faster if a single active is applied.
In fact, the data analysis also found that an economic yield response was unlikely from any fungicide application, including multiple sprays, in low rainfall environments.
Even in a wetter year such as 2022, tillering applications weren’t necessary to manage SFNB in medium-low rainfall areas. In a trial at Nungarin, a single fungicide application at tillering briefly lowered SFNB, but by grain filling stage, there was no difference in disease levels compared to untreated plants. Even a triple fungicide regime (tillering, extension and booting) didn’t result in a higher grain yield over an untreated (Figure 2) for Spartacus CL or Maximus CL, although overall the Maximus CL yielded more than Spartacus CL.

Variety resistance is the simplest way to manage all diseases and there are now new varieties that growers can use to avoid Spartacus CL’s Susceptible to Very Susceptible (SVS) rating for SFNB (Table 1). Even a small step up to the rating of Moderately Susceptible to Susceptible (MSS) will noticeably reduce SFNB levels in crops. To see which barley varieties are susceptible to STNB and NTNB refer to DPIRD’s 2023 WA Crop Sowing Guide - Barley.
For further information on symptoms and management of blotches see DPIRD’s Managing spot type net blotch in continuous barley and Managing net-type net blotch of barley in Western Australia pages.
For more information on blotches contact Plant Pathologists Andrea Hills, Esperance on +61 (0)8 9083 1144, Kithsiri Jayasena, Albany on +61 (0)8 9892 8477, Kylie Chambers, Northam on +61 (0)8 9690 2151 or Jason Bradley, South Perth on +61 (8) 9368 3982.
Article authors: Andrea Hills (DPIRD Esperance), Jason Bradley (DPIRD South Perth), Geoff Thomas (DPIRD South Perth) and Kylie Chambers (DPIRD Northam).
Scald on barley
- Albany region

James Lydon (Nutrien) recently found minor scald symptoms in Laperouse barley in the Albany region. The crop had been sown in late April and was at growth stage Z31.
Symptoms
Scald symptoms first appear as oval grey-green spots on leaves. The spots become elongated, often diamond shaped and bleached with a distinctive brown margin. Lesions usually join to form necrotic areas and the entire leaf withers and dies.
Disease may be widespread across a paddock arising from infested stubble or start as 'hotspots' and rapidly spread, favouring thick crop areas where humidity is high.
The fungus is carried from season to season on infected barley and wild grass residues, regrowth barley or infected seed that acts as an initial source of infection. Early sown crops develop higher levels of scald as they may be exposed to the heaviest release of spores from infected residues.
Management
There are a range of foliar fungicides that can provide effective control of scald.
The disease can also be managed through choice of more resistant varieties and avoiding double cropping or close proximity to last year's infected barley stubbles. Use of disease-free seed and seed or fertiliser applied fungicides at sowing will also limit infection at early growth stages.
Barley foliar fungicide information can be found at DPIRD’s Registered foliar fungicides for cereals in Western Australia.
Further information
For more information refer to DPIRD’s Diagnosing barley scald page.
For more information contact Kithsiri Jayasena, Plant Pathologist, Albany on +61 (0)8 9892 8477, Kylie Chambers, Northam on +61 (0)8 9690 2151, Geoff Thomas, Plant Pathologist, South Perth on +61 (0)8 9368 3262, Andrea Hills, Plant Pathologist, Esperance on +61 (0)8 9083 1144 or Ciara Beard, Plant Pathologist, Geraldton on +61 (0)8 9956 8504.
Article author: Cindy Webster (DPIRD Narrogin).
Register to attend DPIRD’s 2023 crop disease and insect identification workshop
DPIRD are offering their annual Pest and Disease Identification Course again this year (with the usual general diagnostic, root and foliar pathology and entomology components).
Date: Tuesday 22 August to Thursday 24 August 2023
Venue: DPIRD’s South Perth office, 3 Baron-Hay Court, South Perth WA
Insect identification and integrated management on Tuesday 22 August followed by disease identification on Wednesday 23 August and Thursday 24 August. An additional weed ID seminar can be offered if demand exists.
This course is designed mainly for agronomists, growers and other grains industry representatives to improve disease and insect identification skills relevant to broadacre crop production in WA.
The course has a practical and “hands on” training approach, professional and experienced presenters and the valuable take home resource materials.
As usual participants can register to attend either or both components.
The cost for attending the course is $400 for attending all 3 days; or $300 for the two-day disease component; or $150 for the one-day insect component. This cost includes a course reference book and catering for each day.
The entomology training is co-funded by GRDC through the IPMforGrains project, which is delivered by the National Pest Information Network (Cesar Australia, DPIRD, QDAF, NSW DPI and SARDI).
Numbers are limited for the training days and enrolments close on Friday 4 August 2023.
For further details, and to register your interest in attending, contact Plant pathologist Geoff Thomas, South Perth on +61 (0)8 9368 3262 or +61 (0)428 947 287.
Article author: Geoff Thomas (DPIRD South Perth) and Cindy Webster (DPIRD Narrogin).






