This issue of Protecting WA Crops will investigate the impact of liming on weed growth, rhizoctonia root rot activity and crop susceptibility to Root Lesion Nematodes (RLN).
On a national scale, annual crop yield loss due to competition from surviving in-crop weeds is estimated to cost Australian growers $278 million. Competition from a healthy crop remains one of the most cost-effective methods of weed control. For areas with acidic soils, improved crop competition could be achieved by applying lime.
The DPIRD soils team investigated the relationship between liming and weed control over multiple seasons and the key finding was that liming could improve a crop’s ability to compete against weeds. Trial plots that were limed in 1991 and 2000, and subsequently had increased soil pH, increased barley yield and reduced annual ryegrass biomass in 2009.
When soil acidity is regulated following the application of lime, the growing environment is improved and the plants are healthier, grow more efficiently and better resist the effect of weeds, pests and disease.
This newsletter issue follows on from Protecting WA Crops Issue 20 Impacts of mechanical soil amelioration on weeds, soilborne nematode pests and fungal pathogens.
Liming reduces weed growth
The effect that application of lime and incorporation of lime would have on the growth and seed set of annual ryegrass within a crop was investigated by a team of DPIRD researchers, led by Dr Catherine Borger in 2018. Two field sites were located at Merredin (lime was applied in 1994) and Wongan Hills (limed in 2014).
At a glance
- Historical lime application reduced the density and biomass of annual ryegrass in wheat crops, as measured in the 2018 season.
- Like crops, annual ryegrass does not like acidic conditions and grows best when the soil pH is close to neutral.
- Given that annual ryegrass growth was reduced in these field trials, it is likely that application of lime and the subsequent increase in soil pH improved the growth and competitive ability of wheat.
- Altered soil chemistry from lime application may also have influenced herbicide performance, although other work within DPIRD has not found an interaction between liming and efficacy of various herbicides.
- Improved crop competition has previously been shown to reduce annual ryegrass growth and seed production.
Merredin Research Facility in 2016 on a yellow sand
Field trial treatments included a continuous wheat (2016-2018) or wheat-chemical fallow rotation (i.e. wheat in 2016, chemical fallow in 2017 and wheat in 2018), lime applied at various rates of 0, 2, 4 or 6 t/ha and initial cultivation to incorporate the lime (with offset discs to a depth of 15cm). Annual ryegrass density was assessed on 12 July 2018 and annual ryegrass biomass was harvested on 10 October 2018.
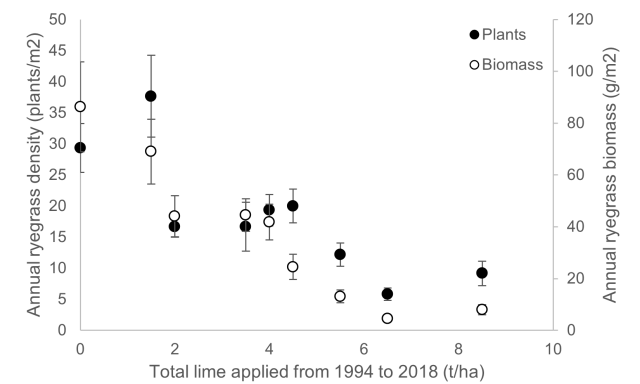
Annual ryegrass density and biomass were both reduced by increasing rates of lime (Table 1).
| Annual ryegrass | Lime (t/ha) | P | |||
|---|---|---|---|---|---|
| 0 | 2 | 4 | 6 | ||
| Density (plants/m2) | 14.2 | 11.3 | 4.1 | 1.8 | <0.001 |
| Biomass (g/m2) | 26.8 | 30.9 | 10.8 | 11.4 | 0.008 |
The wheat-chemical fallow rotation had lower annual ryegrass density than the continuous wheat rotation (1.0 and 17.9 plants/m2) and lower annual ryegrass biomass (3.6 and 46.2 g/m2). Incorporation of lime in the continuous wheat rotation reduced annual ryegrass density (12.1 plants/m2) compared to the non-incorporated treatments (25.1 plants/m2).
In the wheat-fallow rotation there was no effect of incorporation, due to very low weed density (1.5 and 0.6 plants/m2) in the incorporated or non-incorporated treatments in the fallow treatments.
Wongan Hills established in 1994 on a yellow sand
Lime was applied at various rates in 1994, further lime applied to half of the plots in 1998 and final lime applications made in 2014.
The trial was sown to wheat in 2018. Initial annual ryegrass density was low (0 to 5 plants/m2) due to a dry autumn, but cohorts emerged later in the season. On 19 October 2018, annual ryegrass density was recorded, before harvesting annual ryegrass biomass.
Read the full Grains Research Updates paper describing this work.
The effect of liming on Rhizoctonia root rot
In WA it has been observed that there is an increase in rhizoctonis root rot disease (caused by Rhizoctonia solani AG8) in trials with +/- lime treatments. Some farmers have also noted that there were more R. solani issues in limed paddocks.
DPIRD’s Daniel Huberli has been involved in a national project exploring the impact of lime application on rhizoctonia root rot.
At a glance
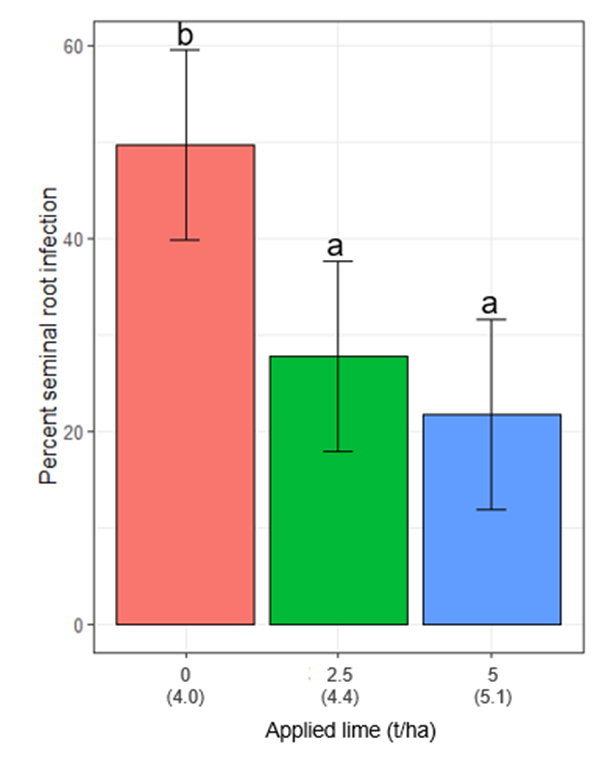
- Lime reduced the R. solani infection of seminal barley roots by 22% and 28% for the 2.5 and 5.0 t/ha applied lime treatments.
- The large beneficial impact of lime application on reducing disease was reflected in a corresponding increase in grain yield. Barley yielded an additional 3.1 t/ha when 5.0 t/ha lime had been applied.
In 2020, an experiment on the interaction between rhizoctonia root rot (R. solani) and lime were established in Wongan Hills, WA.
The experiment was situated on areas where known quantities of lime had been applied in 2012. A Spartacus barley crop was sown over the site and R. solani inoculated over the area.
The average pH levels at the 0-10 cm depth of soils collected in June 2020 were 4.0, 4.4 and 5.1 for the 0, 2.5 and 5.0 t/ha lime applied in 2012, respectively. As there was good differentiation between the lime applied in 2012 and the pH levels in the soil in 2020, the area was oversown with Spartacus barley and half of the plots were inoculated with R. solani to determine the effect of lime on disease incidence and severity.
Both lime and R. solani inoculum had a real effect (p<0.05) on seminal root infection. Lime reduced the R. solani infection of seminal roots by 22% and 28% for the 2.5 and 5.0 t/ha applied lime (Figure 2), respectively, while R. solani increased the infection by 12% when compared to the untreated control. For the uninoculated plots seminal root infection was 27% which was higher than expected as the start of the season had a low level of R. solani detected at the site.
The season may have increased the R. solani inoculum substantially or some other soilborne pathogen such as Pythium (also detected at the site at the beginning of the season) contributed to higher symptomatic seminal roots. Lime had no effect on the proportion of crown root infection. Applying R. solani inoculum significantly (p<0.001) increased the proportion of crown roots infected by 35% when compared to the uninoculated plots.
The large impact of lime on disease was also reflected in the large significant (p<0.001) increase in grain yield of 3.1 t/ha in the 5.0 t/ha lime treatment (Figure 3). As expected, both lime treatments had a significant impact on grain quality. Screenings were reduced by more than 8.7%, test weight was increased by over 1.1 kg/L, and 1000 seed weight was increased by over 2.4 g when both lime treatments were compared to the untreated. R. solani did not have a significant effect (p>0.05) on grain quality.
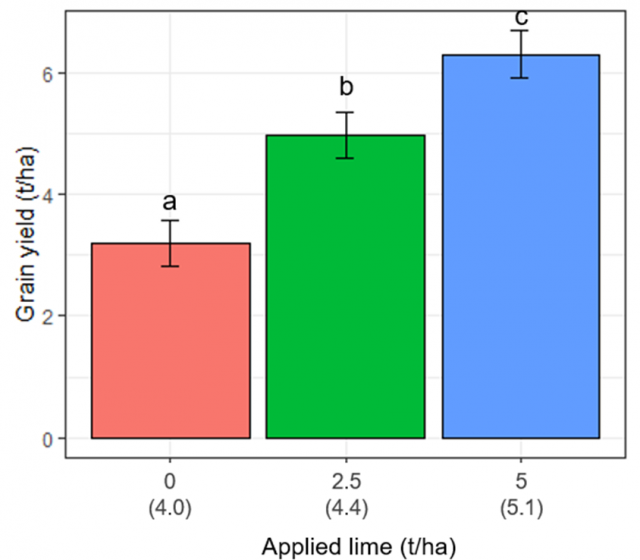
In WA, observations that liming increases rhizoctonia root rot in growers' paddocks instigated this single trial in 2020. The changes in pH as a result of liming showed that seminal root infection by R. solani was significantly reduced when the two treatments were compared to the nil lime treatment, but disease in crown roots was not affected by lime. The large benefit of the lime treatments was the significant grain yield increase of over 3 t/ha in the 5.0 t/ha lime treatment, which is more than double the unlimed nil treatment.
Observations of increased disease from liming appear to be on early limed paddocks, which may explain the differences in the trial findings which were on a site with equilibrated pH levels from liming treatments applied in 2012. Future trials should investigate early liming in conjunction with nutrition to determine whether the interaction predisposes crops to soilborne diseases.
Does soil acidity impact crop resistance to root lesion nematodes (RLN)?
Acid soils are prevalent across Western Australia’s broadacre cropping areas and are known to impact nutrient uptake and increase crop vulnerability to pests and pathogens. As more than 65% of broadacre cropping paddocks are infested with root lesion nematodes (RLN), a pest of cereals, pulses and oilseeds - the effects of pH on RLN multiplication were assessed by a team led by DPIRD’s Sarah Collins in glasshouse (2014) and field experiments in Northam, Goomalling, (2015 and 2016) and Wongan Hills (2017) for common WA species Pratylenchus quasitereoides and Pratylenchus neglectus.
At a glance
- Acidic soils reduced the resistance of crops, particularly to RLN species P. quasitereoides. Higher final populations of P. quasitereoides were recorded for seven of the nine wheat, barley and lupin varieties grown in low pH soil compared to the same varieties grown in the same soil type that was amended with lime to a moderate pH. In field trials liming soil reduced RLN species P. quasitereoides multiplication in barley.
- P. neglectus results were less conclusive. Liming acidic soil reduced barley and wheat resistance to P. neglectus but multiplication increased in a field trial when soils were ameliorated with lime.
- For canola there was no effect of pH on multiplication of P. quasitereoides or P. neglectus in the field or glasshouse trials.
- It is thought that there is a direct relationship between soil pH and plant stress which leads to an increase in multiplication of RLN. More research is warranted to obtain conclusive results for both RLN species common to WA soils.
Glasshouse trials investigated whether soil acidity negated crop and variety resistance and increased the ability of P. neglectus and P. quasitereoides, the most common RLN species in WA broadacre cropping, to multiply on commonly grown crops.
The first experiment investigated the potential impact of acid soils on resistance of wheat, barley, canola and lupin crops to P. neglectus or P. quasitereoides. Varieties with a range of RLN resistance were tested. Soil came from a pH experimental trial paddock in Wongan Hills, where limed and unlimed strip plots are maintained (pHCaCl 6.7 & 5.1 respectively). Pots were inoculated with RLN a week after germination and grown for 10 weeks in the differing pH soils in a glasshouse. Nematodes were extracted from the entire roots and counted microscopically.
The second experiment investigated the impact of acid soils on P. neglectus resistance of wheat cultivars. Soil from two field sites, with adequate (pHCaCl 6.4) and low pH (pHCaCl 4.7) were utilised. Wheat cultivars considered more resistant (Wyalkatchem, Yenda and Tamaroi) and susceptible (Calingiri, Wylie and Brookton) to P. neglectus were assessed using similar methods to Experiment 1.
Limed field trials from DPIRD’s ‘More profit from crop nutrition’ project at Goomalling and Northam were naturally infested with P. quasitereoides, so we took the opportunity to assess them for RLN levels and multiplication over 2 seasons (2015/16) when barley or canola was sown. A third trial, sown to mace wheat in Wongan Hills and infested with P. neglectus was assessed in 2017. Lime sand was incorporated with 0, 2.5 and 5 t/ha in 2012 at Goomalling and Northam and 0 and 4 t/ha in 2017 at Wongan Hills.
Results and discussion
P. quasitereoides populations were significantly higher in barley, lupin and wheat crops grown in acid soil, and in barley only for P. neglectus (Figure 4a and 4b.), compared with RLN populations extracted from crops grown in the same soil type maintained at moderate pH. In canola, there was no effect of pH on multiplication of P. quasitereoides or P. neglectus.
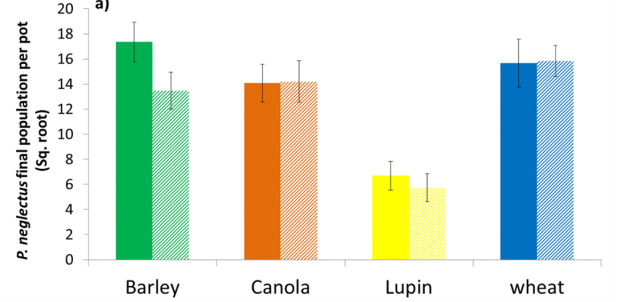
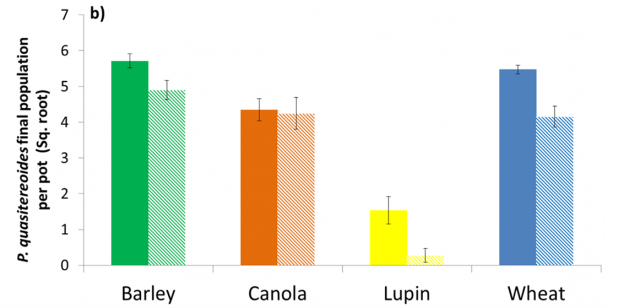
It is thought that there is a direct relationship between soil pH and plant stress which leads to an increase in multiplication of RLN. Canola is known to be tolerant of soils at the pH tested (5.1) so may not have been stressed and thus there was no increase in RLN multiplication.
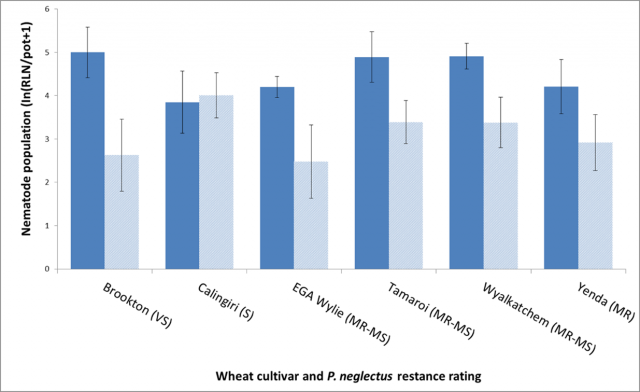
When wheat cultivars with a range of resistance to P. neglectus were grown in low and adequate pH, soil nematode multiplication was higher in the more acid soil compared to moderate pH soil in five of the six varieties tested, regardless of inherent cultivar resistance (Figure 5). The relationship between acid soils, and resistance to P. neglectus was not conclusive. In Experiment 1 there was a significant (p<0.05) increase in final numbers of RLN in two of the nine varieties tested, in four crops in low pH soil, compared to those grown in the same soil type maintained at moderate pH.
Field trials reinforced that barley resistance to P. quasitereoides is increased by mitigating soil acidity. At Goomalling, lime amendment of 2.5 and 5 t/ha inhibited P. quasitereoides multiplication in a barley crop compared to non-amended soil (p<0.05). At the Northam trial P. quasitereoides multiplication during the 2015 season was very high at the site but decreased significantly with increasing lime application (Figure 6), but not in 2016 when a canola crop was grown.
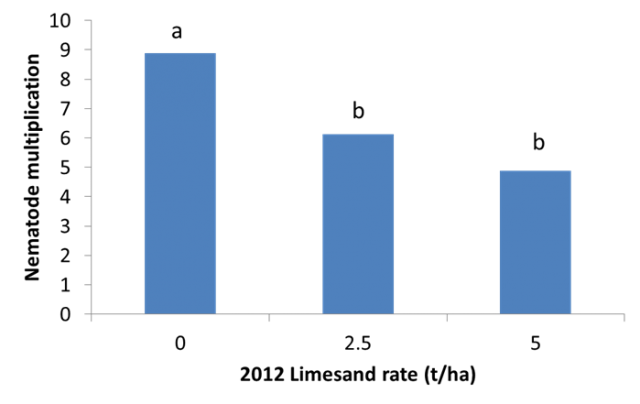
Our experiments established that some of WA’s commonly grown crops, wheat, barley and lupin, are less susceptible to P. quasitereoides in soil that had been limed to reduce acidity. The effect of increasing pH on resistance of these crops to P. neglectus has not been clearly established. Liming acidic Western Australian broadacre cropping soils, may also decrease RLN multiplication and the potential detrimental effect of RLN, on crop growth and yield.
 Meet the team - Bowen Zhang
Meet the team - Bowen Zhang
Bowen joined DPIRD’s Northam crop protection group in February 2022 to continue working on a three-year project, which aims at investigating the impact of soil amelioration on weed ecology and control.
Before joining DPIRD, Bowen had been studying agricultural science at UWA since 2017. He was inspired to start his career in weed research after attending various lectures during his bachelor’s degree. He has worked alongside AHRI researcher Dr Roberto Busi on his master’s thesis and successfully identified the novel resistance of wild radish to HPPD-inhibiting herbicides.
This masters project gave him the opportunity to obtain great experience working in glasshouses and labs. After completing his master’s degree in 2021, Bowen continued to work at AHRI as a research assistant to help conduct 2021 AHRI herbicide resistance test. Through this he gained a comprehensive knowledge and learnt updated resistance information with various weed species in the WA wheatbelt. Working with soil is comparably a new field in Bowen’s career, but he is looking forward to collaborating with other DPIRD researchers and growers to develop new weed management approaches using soil amelioration practices.
 PhD Scholar - Maninder Kaur
PhD Scholar - Maninder Kaur
Mrs Maninder Kaur, a PhD student from Murdoch University, with a Grains R&D Postgraduate Scholarship from DPIRD, is investigating cold plasma technology to treat Fusarium graminearum, a primary fungal pathogen of Fusarium head blight disease in cereals and associated mycotoxins, under the supervision of Dr Kirsty Bayliss from Murdoch University and Dr Daniel Huberli from DPIRD.
Cold plasma is an ionised gas, that does not leave any chemical residues on treated grain. It is an inexpensive and environmentally friendly technology with low maintenance requirements and works at ambient temperatures. The first phase of her research has shown promising results, with cold plasma reducing the growth of pure cultures of Fusarium species isolated from wheat and barley grain, and on mould contaminated grain. Her tests on germination and quality analysis of treated wheat grain also indicate that cold plasma does not alter the germination and quality of grain. She is now testing the effect of cold plasma to detoxify mycotoxins. Her focus is to minimise the agriculture industry's reliance on chemicals while maintaining the product's quality.
Maninder has recently been awarded a Fulbright scholarship. As a Fulbright scholar, she will continue her work on cold plasma to understand the pathogen's pathogenicity, defence response, and interaction with cold plasma treatments. This project will help identify the genomic mutations in F. graminearum induced by cold plasma, which could be used to predict the changes in the fungal virulence against the host plant.
This project will assess an innovative and environmentally friendly approach to managing Fusarium graminearum, a concerning pathogen for both the United States and Australia. With increasing consumer demand for chemical-free and safer food, this project could potentially lead to greater economic returns in the long term for both nations.
 Grains Convo Podcasts
Grains Convo Podcasts
Did you know that DPIRD has a new podcast series? Grains Convo is published twice a month and explores areas of crop protection (weeds, pests and diseases), as well as other grains research projects undertaken by the Department.
Listen to the podcasts on the GGA website, or on Apple podcasts and Spotify.
Crop protection related podcasts already available include:
- Native budworm
- Managing sclerotinia
- Turnip yellows virus and its vector green peach aphid
- Australian plague locusts and grasshoppers
- Emerging weeds – seed bank ecology of emerging weeds
- The effect of a green bridge on root lesion nematodes (RLN)
- New technologies for monitoring crops for diseases and insects
- Managing snails in broadacre crops
- How the PestFacts WA service can help you
- Disease risk forecasts for blackleg in canola and blackspot in field peas

GRDC Research Updates Perth - March 2022
The GRDC research update this year was a virtual event. There were several contributions to the program from researchers in DPIRD’s Crop Protection team. A full list of the crop protection presentations (some with supporting papers) is available here.
- Ciara Beard and Pippa Michael – Understanding and managing sclerotinia in lupins
- Catherine Borger - Best rotations for barley grass management
- Miranda Slaven - Electric weed control in Australia
- Anna Hepworth - Spraying for yellow leaf spot in wheat - will you lose money?
- Bowen Zhang - Efficacy of pre-emergent herbicides on renovated soil
- Alex Douglas and Dave Nicholson - The biology and control of matricaria and marshmallow - can we run down the seed bank?





